Anti-clogging Chemicals May Cause Fouling
Civil and environmental engineering professor Helen Nguyen has found that water-softening additives may increase the risk of pathogen release into drinking water by weakening the grip that bacteria have on pipe interiors. (Credit: L. Brian Stauffer, University of Illinois, https://news.illinois.edu/view/6367/675371)
Drinking water management plans in many cities use chemical softening agents to reduce mineral buildup and keep water flowing inside the plumbing. Recent research reveals that these additives might make plumbing a much more slippery situation, not just for water, but for pathogenic bacteria such as those that cause Legionnaires’ disease, too. Biofilms grow inside pipes that carry water, attaching themselves to buildups of mineral scale.
“Mineral scales are precipitates from chemical compounds in drinking water,” explains Dr. Helen Nguyen, a professor of civil engineering at the University of Illinois, and co-author of the recent study. “Biofilms are made up of microorganisms and chemicals excreted by the microbes. These microbes are also from drinking water. Chemical precipitates can be found within the biofilm matrix.”
Scaling on pipes protects water quality, in some cases, such as when pipes are lead or have lead soldering; in those cases, mineral scales are very important to prevent leaching lead or iron oxides into drinking water. However, if scales are not controlled, they will completely block pipes.
The problem, as Dr. Nguyen explains, is controlling scale build-up in the plumbing systems inside a building so that the water delivered to customers is safe.
“It is a challenge for drinking water treatment plants to maintain a delicate balance in scale control through a very large distribution system, including the water main, the service line and privately owned plumbing systems,” details Dr. Nguyen. “The question is how to control scale build-up so that we do not create unintended consequences. Our study has found that polyphosphate, commonly used to control scale, could promote the growth of biofilms on the plumbing pipes. In addition, we found that thicker and softer biofilms grown from water with polyphosphate compared to the biofilms from the same water but without the added polyphosphate.”
This is important because in a previous study the team found that softer biofilms reduced the ability of residual disinfectants such as free chlorine or monochloramine to inactivate Legionella pneumophila released from biofilms—a dangerous bacteria that can cause lung infections.
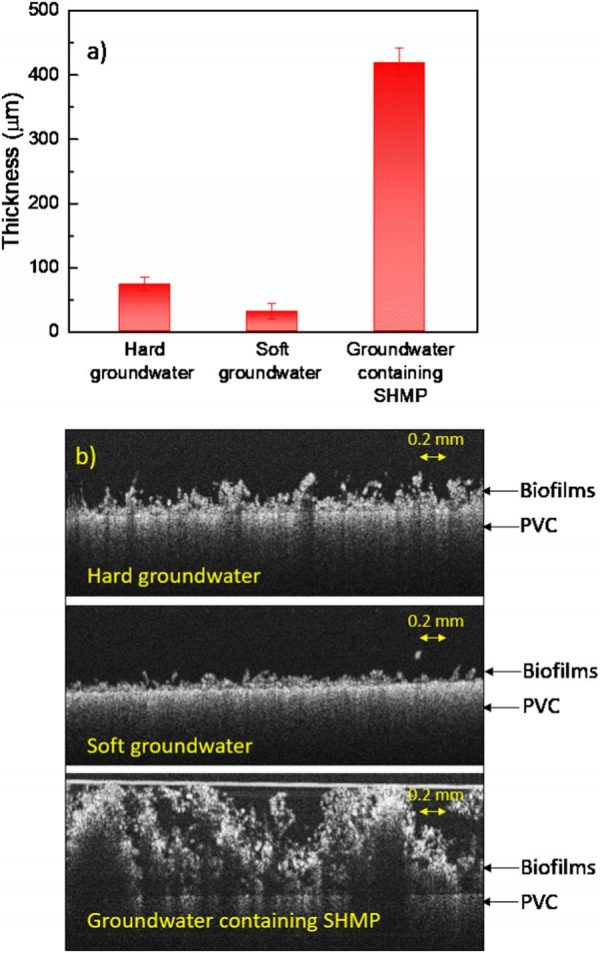
a) Average thickness and b) selected OCT images of biofilms developed from hard groundwater, soft groundwater, and groundwater containing SHMP. (Credit: Shen et al., https://www.nature.com/articles/s41522-018-0058-1/figures/3)
“Softer biofilms are easier to break during exposure to shear stress caused by water flow—for example when we open the tap,” Dr. Nguyen describes. “Pieces of broken biofilms could contain pathogens such as Legionella pneumophila. In addition, biofilm materials surrounding these pathogens could cause depletion of the disinfectant; as a result, the effectiveness of the disinfectant for pathogen control is reduced.”
Biofilms in the lab
The team used magnetomotive optical coherence elastography to measure the softness and thickness of lab-grown biofilms during the experiments. The tool is typically used to measure cancer tissue strength, but the team, in this case, adapted the technique for quantifying the strength of biofilms they grew inside PVC pipes using local groundwater.
“We focused on physical properties of biofilms, including thickness, roughness and stiffness,” remarks Dr. Nguyen. “The biofilms were grown using groundwater after granular filtration. This groundwater is the same water source used in Urbana-Champaign IL. We simulated different scenarios by adding antiscalant and anticorrosion inhibitors to this groundwater and grew biofilm over months from these waters. After that, we characterized the biofilms.”
Dr. Nguyen and the team have spent the last nine years working on this problem. They have learned a great deal about it, but there are still many remaining questions.
“We are currently trying to understand what anticorrosion and antiscalant chemicals cause the lowest biofilm growth and biofilm breakage,” states Dr. Nguyen. “Phosphate-based chemicals such as polyphosphate, orthophosphate, and mixtures of these two are the most commonly used. However, we are looking into other non-phosphate based chemicals. We are also studying how to remove biofilm in-situ using a new technology of microplasma jet arrays.”
One of the important takeaways from the research is that a better understanding of water chemistry is a more fruitful source for solutions than a campaign to eliminate all microbes from water sources—or a drive to replace all infrastructure.
“Biofilm is unavoidable,” concludes Dr. Nguyen. “What chemical we add to drinking water to prevent corrosion and scaling plays an important role in the safety of drinking water at taps.”
Top image: Civil and environmental engineering professor Helen Nguyen has found that water-softening additives may increase the risk of pathogen release into drinking water by weakening the grip that bacteria have on pipe interiors. (Credit: L. Brian Stauffer, University of Illinois, https://news.illinois.edu/view/6367/675371)




Matthew Mabey
October 15, 2018 at 12:06 pm
One thing that is missing from both this summary and the published paper is a description of how the biofilms were grown. It isn’t stated what the history of water flow in the pipes test was. I would think that the pipe flow would have a tremendous impact on the nature, composition , structure, and strength of both the scale and biofilm that forms in a pipe. Thus, not stating the flows used for their study seems like a significant omission.
Burnice Bauch
April 19, 2023 at 11:05 pm
Wow, I never realized that using anti-clogging chemicals could actually cause fouling in the long run. This is such an eye-opening article, and I really appreciate you taking the time to share this important information. It’s clear that your team is really dedicated to providing valuable insights and helping people make more informed decisions. Keep up the great work!