Eureka! Scientists Using Chemistry to Filter Silver Nanoparticles Out of Wastewater
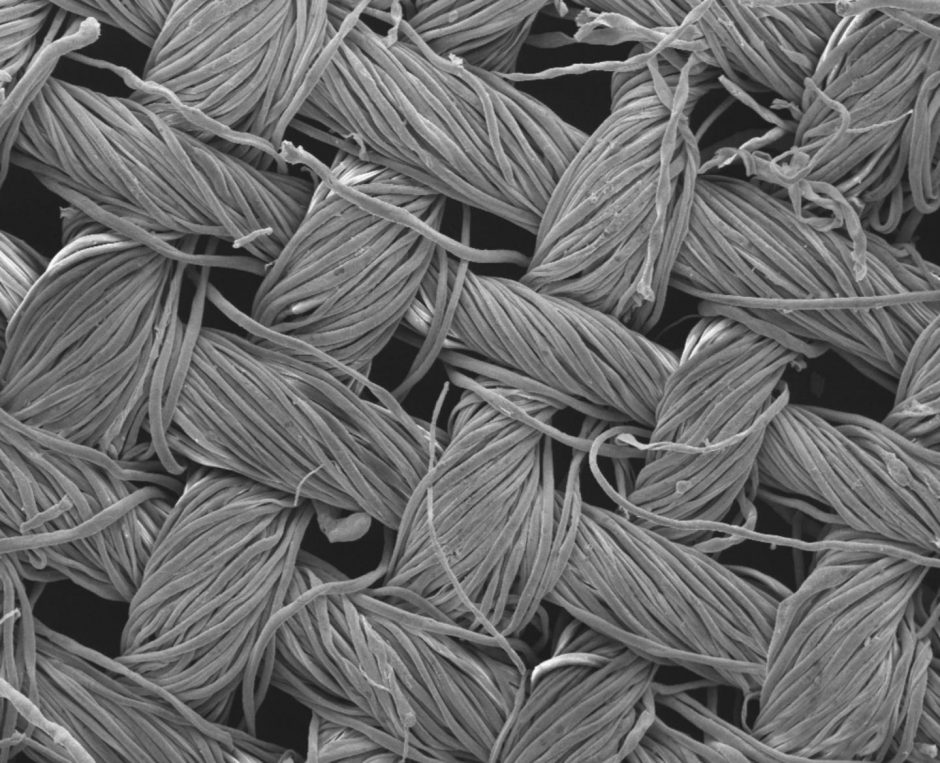
Cotton textile covered with nanostructures invisible to the naked eye. Image magnified 200 times. (Credit: RMIT University)
Silver nanoparticles are a kind of miracle additive for anyone who has ever been responsible for laundering the smelly clothing of, for example, active teenagers or athletes. These nanoparticles deliver silver ions that promote lysis, cellular breakdown, making them toxic to the bacteria that cause body odor. This means that clothing laced with silver nanoparticles can be worn by even the sweatiest, smelliest athlete without taking on those odors.
However, that’s not the end of the story. In fact, this is really just the first chapter in the story of the life of silver nanoparticles. As the clothing is washed, some of the silver nanoparticles can be washed away in the wastewater, moving on to the next chapters of their existence. Unfortunately, what happens from there on out is poorly understood; scientists aren’t sure how long these nanoparticles persist in the environment, where they go, or what they do.
What we do know is problematic. Silver nanoparticles can kill odor-causing bacteria, but that also means they can kill other types of bacteria, including those that affect the function of wastewater treatment plants. They can also prove toxic to various aquatic organisms.
Dr. Sukalyan Sengupta is a Professor of Civil and Environmental Engineering at the University of Massachusetts, Dartmouth. His research interests include physicochemical treatment processes and process modeling of water and wastewater treatment systems. He is well aware of the problem silver nanoparticles can pose in the environment.
“The silver in laundry wastewater can enter the environment through various routes,” Dr. Sengupta explains. “Silver-containing wastewater is transported to wastewater treatment plants (WWTPs). There it can potentially harm the bacterial treatment processes, resulting in compromised performance of the WWTP. Moreover, in a WWTP, the silver ends up in biosolids, which is typically applied on land for enhanced agricultural productivity. The silver may be taken up by plants, or may leach into the groundwater and surface water sources, where it can harm a number of aquatic life forms such as zebrafish, rainbow trout, etc. There is also a reported risk of silver entering our food chain via plants and aquatic organisms. In humans, the ingested silver, depositing beneath the skin and pupil, leads to a condition called Argyria, in which the affected person develops a bluish appearance.”
Perhaps one of the greatest concerns is that the totality of the hazard posed by nanosilver in the environment is still not fully known—especially since there is no sign of the use of silver-based antimicrobial textiles waning.
“However, many studies indicate the ecotoxicological risks of silver nanoparticles in different scenarios,” Dr. Sengupta confirms.
Unfortunately, removing nanoparticles from laundry-generated wastewater isn’t a simple fix. This is primarily because the silver is present in very low concentrations, it’s not clear which exact types of silver is present in the water, and there are high concentrations of other ions competing with the silver.
Panning for silver nanoparticles in wastewater
Dr. Sengupta and fellow researcher Tabish Nawaz developed an ion-exchange technology which is highly selective for silver, but until now they had not researched the role detergent chemistry might play in the process. Their latest findings explain how detergent chemistry and silver nanoparticles interact in the context of their “panning” removal process.
Sengupta and Nawaz discovered that silver exists primarily as a positively charged ion, meaning that under specific conditions, it will interact with the negatively charged ions in several detergent compounds. The team used a a thiol-group functionalized ion-exchange resin in a fixed-bed column to “pan” or separate the silver out from laundry wastewater, and this allowed them to recover up to about 99 percent of the silver nanoparticles, depending on the concentration and pH of the competing ions.

Close-up of the nanostructures grown on cotton textiles by RMIT University researchers. Image magnified 150,000 times. (Credit: RMIT University)
“The silver removal by the thiol functionalized resin, Ambersep GT74, happens via ion-exchange, where the resin (preloaded with Na+ & H+) selectively takes up Ag+ from the background matrix of detergent solution and competing cations such as Ca2+, Mg2+, and Na+, which are in at least an order of magnitude higher concentration,” Dr. Sengupta describes. “The uptake mechanism of silver is silver – sulfur complexation, where sulfur in the thiol group coordinates Ag+ forming a highly stable silver – thiol complex.”
The “panning” analogy comes from the physical manifestation of the process.
“We first prepare a packed bed of the resin, and the influent solution containing silver with the background detergent matrix is passed in a downflow mode through the column,” Dr. Sengupta details. “We then adjust the flow rate to provide an adequate contact time for effective silver removal. The bed selectively takes up silver and passes the remaining solution as a silver-free effluent solution. Once the bed is saturated, the regenerant solution, acidified thiourea, is passed to leach silver from the bed, and generate a silver-rich, spent regenerant stream, which is then subsequently processed to recover the silver as silver nanoparticles.”
Although the results were impressive, adding products such as bleach and fabric softener negatively affected the resin’s efficiency.
“Recovering silver from laundry wash solution requires preventing silver from interacting with the detergent components,” Dr. Sengupta cautions. “The builders (zeolites, polycarboxylates) and bleaching agents (perborate) negatively affect silver availability in the solution and the resin performance. Manipulating the detergent solution conditions so that these components free up silver ions is central to this process. pH adjustment or Ca2+ availability is critical for an effective silver recovery scheme.”
The team tested the resin and the regenerant solution with detergent components over five cycles, without any significant loss in their performance.
“Since we needed to publish the results as quickly as possible, we did not carry out the reusability study beyond five cycles,” comments Dr. Sengupta. “However, we know that ion-exchange resins can be used for hundreds of cycles without any deterioration in their performance.”
This suggests a fairly exciting potential for commercialization in the future—and, more importantly, widespread ability of everyday people to use the process in their washing machines at home, reducing global environmental risk posed by silver nanoparticles. The team has already filed for a US patent on their discovery, and envisions an ion-exchange cartridge that can be fitted to home washing machines, although their more immediate plans include testing the technology at large wash water flow rates such as those achieved in commercial laundromats, hospitals, airports, and hotels.

Silver ions delivered by nanoparticles to bacteria promote lysis, the process by which cells break down and ultimately die. (Credit: Zongming Xiu/Rice University)
What is next for the team and this line of research?
“We are studying certain physical parameters for optimum silver recovery,” Dr. Sengupta states. “So far, our work involved developing and optimizing the process chemistry model. The next area of research and development pertains to physical parameters like mode of contacting and influent flow rate.”
Like most other developments involving resource recovery, this one will require commitment from the community. Dr. Sengupta hopes that we all take an active role in making new technologies like this one part of our wastewater treatment standard.
“As technologists, we can develop environmental friendly and economic solutions,” Dr. Sengupta explains. “However, fulfilling the social aspects requires active participation of the community. For a sustainable future, we appeal to the readers to initiate a conversation within their societies for larger integration of resource recovery methodologies in waste management and treatment practices.”
Top image: Cotton textile covered with nanostructures invisible to the naked eye. Image magnified 200 times. (Credit: RMIT University)




Pingback: Panning for silver nanoparticles in your clothes washer | FrogHeart