Fostering a Notion of Environmental Antibiotic Stewardship to Fight Drug-Resistance
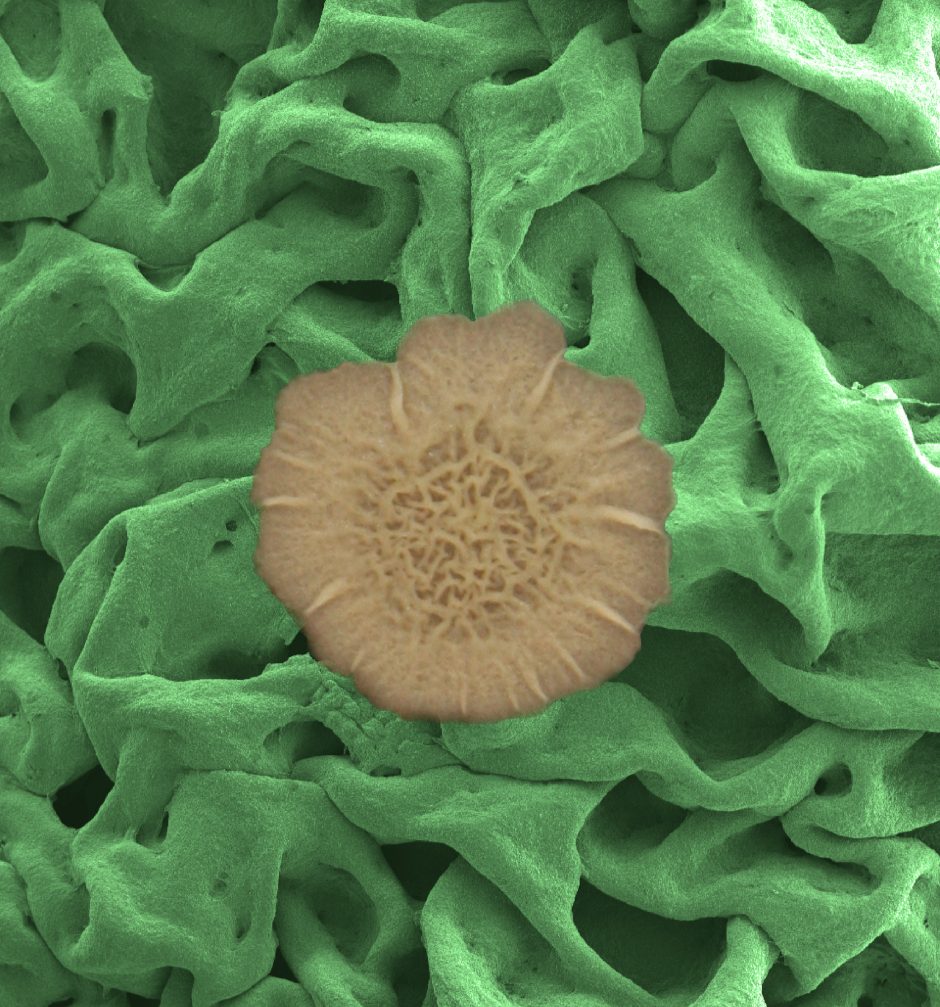
An electron microscope image shows the biofilm surface's resilient meshwork made from proteins and polysaccharides and assembled into a multiscale, hierarchical structure. (Courtesy of: the laboratory of Joanna Aizenberg, Harvard School of Engineering and Applied Sciences)
Infections caused by antibiotic-resistant bacteria affect at least 2 million people and kill 23,000 every year in the US, making them a significant public health problem. In 2015, the White House formally recognized the issue with its National Action Plan for Combating Antibiotic-Resistant Bacteria, prepared by the President’s Council of Advisors on Science and Technology (PCAST). The plan and PCAST report call for “antibiotic stewardship,” which they characterize as the optimized use of antibiotics in both agriculture and human healthcare.
However, although antibiotic resistance is finally on our national radar as a problem, aquatic ecologist Emma Rosi of the Cary Institute of Ecosystem Studies and her colleagues are concerned with a serious flaw in the plans set forth in this existing vision of antibiotic stewardship: the omission of the environmental fate of antibiotics. New research from Dr. Rosi and her team address the problem of rising concentrations of antibiotics in the surface waters of the US; specifically, the team has found that the presence of antibiotics in wastewater is contributing to the development and spread of antibiotic-resistant microbes, and as such is contributing to the problem of antibiotic resistance.
Persistent pharmacological pollution in urban streams
Biofilms in surface waterways are an important component of the ecosystem where bacteria and algae reside. These biofilms are responsible for critical ecosystem functions such as primary production and cycling of nutrients. Dr. Rosi and the team were interested in teasing out whether biofilms and bacteria in urban streams were more likely to exhibit resistance to antibiotics than biofilms and bacteria in suburban streams.
To achieve this goal, Rosi went back to a technique she developed for earlier research. They amended auger with low doses of ciprofloxacin rather than nutrients and allowed the mixture to diffuse through a cellulose sponge. They then deployed these experimental sponges along with control sponges without the ciprofloxacin along an urban/rural gradients of streams.
“The bacteria grow on them, and if they’re sensitive to the drug they don’t grow as well on the one that’s been exposed to the drugs as they do on the unexposed control reference,” Rosi clarifies. “If they’re resistant to the drug, then they grow just as well. And what we’ve found is that in the urban streams they grew the same whether they were exposed to the drugs or not, suggesting functional resilience—they maintain their same ecological function even though they were exposed to a drug.”
Next, the team sequenced the genes of the various bacterial communities, both those that grew well and those that were inhibited by drugs, in both urban and suburban streams. Dr. Rosi and the team wanted to discern exactly which microbes were present, and how functionality was or was not being maintained in each community.
“We found that in the urban stream, even though they maintained their function, there was a significant shift in which microbes were present in the bacterial communities,” Dr. Rosi details. “The communities that were exposed to Cipro, regardless of the site, were indistinguishable from each other. So we think that in these urban streams, bacteria get exposed to pharmaceuticals on a somewhat regular basis because there is leaking infrastructure. The result is the presence of pockets of drug resistant microbes in these streams.”

Eroding stream banks and aging sewer lines contribute to evolving water pollution problems in cities. In this photo from Baltimore, Maryland, a sewage pipe that was originally placed in a stream bed developed leaks, and is now surrounded by a concrete casing. (Credit: Tamara Newcomer Johnson, University of Maryland)
Once some drug resistant microbes exist in the environment, they have the power to create more—some like them, and some different from them.
“Because there are bacteria in rivers and streams developing resistance to antibiotics, the potential for a pathogen to develop resistance through lateral gene transfer is there,” Dr. Rosi cautions. “These bacteria can pass genetic information found on the plasmid of the gene, including resistance to antibiotics, on to other types of bacteria. So it’s sort of a blind spot in our concerns about antibiotic resistance.”
Broader implications for antibiotic stewardship
There are several important takeaways to glean from this work. One is that even with increased vigilance from humans and overuse of antibiotics, to some extent antibiotics are still necessary. This means that antibiotics will still be present in wastewater, until we treat it. This also means that until we have a more functional wastewater and sewage system that is less prone to leaking, antibiotics will leak into the environment.
“These urban streams that we work in around Baltimore are not unusual. There are many urban streams around the US, and all over the world, that receive human waste. In our case it’s in the form of untreated sewage, but even wastewater effluent that’s treated can have antibiotic residues in it. They’re not being removed by the wastewater treatment plants because antibiotics are not considered contaminants.”
And while many bacteria that come into contact with antibiotics are not harmful to humans, the few that are have the potential to cause extensive damage.
“Many of them are not pathogens, but some of them are,” Dr. Rosi points out. “We didn’t look for pathogenic bacteria; we just studied the microbial community in general. If there are antibiotics out there and the bacteria are developing resistance to the antibiotics because they’re being exposed, that has some potential human health risk.”
The way forward will necessarily include both investment into infrastructure repair, and improved wastewater treatment.

Lead author Emma Rosi sampling in a Baltimore stream. (Credit: Heather Bechtold)
“We should do our best to treat our sewage and to prevent sewage getting into the environment with antibiotics in it,” advises Dr. Rosi. “The more we can invest into our sewage and underground infrastructure, and the more we can repair the pipes so that we don’t have leaking sewage, the better. And once we get that waste to a wastewater treatment plant, we need to make sure that we have funds to upgrade our wastewater treatment plants to deal with these types of pollutants. The city of Baltimore where I’ve been working for a long time is working really hard on repairing infrastructure. But they fix one leak and then another leak; it’s old infrastructure, it’s very costly to repair, and there’s often not enough money to support that.”
In the meantime, Dr. Rosi advocates for a sense of antibiotic stewardship that includes attention to the environment.
“The 2015 presidential commission on antibiotic stewardship didn’t mention the environment at all, and yet there are growing numbers of resistant bacteria communities in the environment,” Dr. Rosi adds. “We need to be cognizant of the fact that we are not just using antibiotics in our lives and to treat our livestock; we’re also releasing them into the environment.”
Top image: An electron microscope image shows the biofilm surface’s resilient meshwork made from proteins and polysaccharides and assembled into a multiscale, hierarchical structure. (Courtesy of: the laboratory of Joanna Aizenberg, Harvard School of Engineering and Applied Sciences)




0 comments