Ocean Acidification Disrupting Marine Food Webs
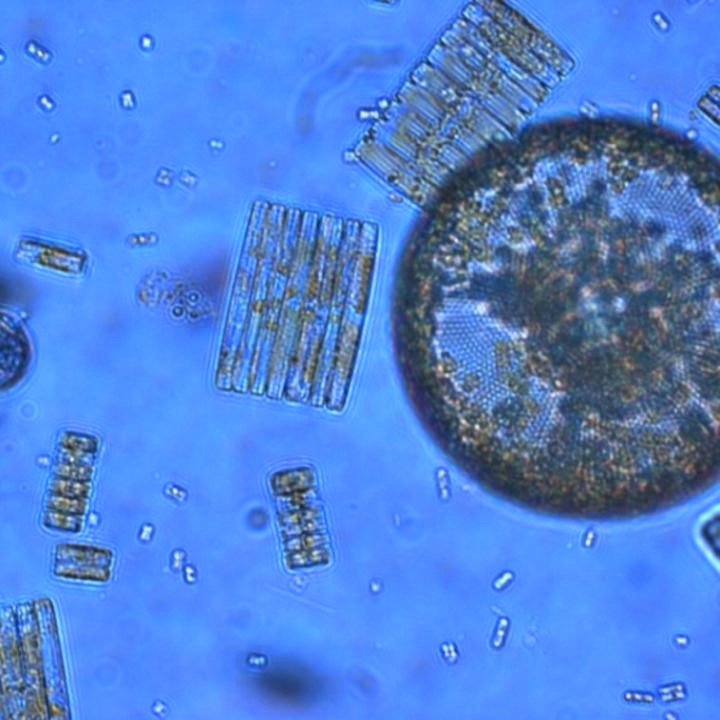
Photosynthetic plankton like these Ross Sea diatoms are key players in the global carbon cycle and form the base of marine food webs, but a new study reveals their ability to acquire iron is highly sensitive to ocean acidification. (Credit: Jeff McQuaid)
Our planet is no stranger to mass extinction events. There have been at least five of them in our collective past. Some scientists believe we are now in the midst of a sixth such event—the first we can call our own human-fueled affair, in what may or may not be the age of the Anthropocene.
Of particular interest as we examine Earth’s past are the possible causes of these mass extinction events. New research from a team led by scientists from Scripps Institution of Oceanography at the University of California San Diego and the J. Craig Venter Institute (JCVI) has revealed a synergistic mechanism phytoplankton use to acquire iron—one that is threatened by atmospheric CO2.
Phytoplankton, iron, carbonate, and atmospheric CO2
Microscopic plants living near the surface of Earth’s oceans around the world—phytoplankton—form the foundation of the marine food web. Phytoplankton also play an important role in the long-term removal of atmospheric carbon dioxide (CO2). Like other types of plants, phytoplankton are primary producers that engage in photosynthesis—in fact, they undertake about half of all of the photosynthesis on Earth. Their need to create organic compounds using the sun’s energy and CO2 dissolved in the water not only sustains the marine food web, but removes CO2 from the atmosphere.
At first blush, this may make the excess levels of atmospheric CO2 produced by burning fossil fuels appear to be a boon to phytoplankton, but this new research proves that the opposite is true. Jeff McQuaid, a Scripps Institution of Oceanography PhD student at the time of the study and now an adjunct scientist at the J. Craig Venter Institute, is the study’s lead author, and corresponded with EM about the research.
“An early operating theory in biological oceanography was that elevated concentrations of CO2 would actually be beneficial to phytoplankton: just like land plants, marine algae need to concentrate carbon prior to converting it into sugars,” McQuaid describes. “The higher the concentration of CO2, theoretically the less energy that plants should have to spend on concentrating it.”
Early experiments along these lines seemed to support this idea, correlating a slight fertilizing effect with more CO2. When scientists exposed phytoplankton in coastal regions to high levels of CO2, they observed changes in the variety of phytoplankton, but no strongly negative effects to phytoplankton productivity or community growth rates.
“However, as those CO2 experiments traveled further out in the ocean to the low-iron, high nutrient regions, something happened: high CO2 seemed to suppress the growth rate and the uptake of nutrients,” McQuaid remarks. “If you added iron to the bubbled phytoplankton, the negative effects of high CO2 went away, so whatever was going on seemed to have something to do with iron in particular.”
With the publication of this research, there is now a clear mechanism connecting iron, CO2, and the ability of phytoplankton to grow.
Finding the linchpin
Initially scientists tackled the question of which waters supported phytoplankton growth, and why.
“With the exception of a few regions, most of the ocean can be thought of as a marine desert, but in key areas like the North Pacific and the Southern Ocean, there are high concentrations of dissolved nutrients in the water, sufficient to support large populations of plankton,” explains McQuaid. “How well the phytoplankton in these regions grow, or phytoplankton ‘productivity’ in bio-speak, more or less determines the rate at which CO2 is removed from the atmosphere, and the main thing limiting growth in these regions is iron.”
As scientists continued to investigate what makes phytoplankton grow, it became clear that there was a missing factor. Although iron was obviously critical for phytoplankton growth and nutrient uptake, iron alone wasn’t the answer. In the past, when the Earth’s climate was cooler, levels of atmospheric CO2 were around 200ppm.

Lead author Jeff McQuaid watches an array of pumps designed to test the effects of high CO2on Ross Sea phytoplankton in Antarctica. (Credit: A. E. Allen)
“There was a lot more terrestrial dust in the atmosphere, and it has been proposed that this dust fertilized these low-iron, high nutrient regions of the earth’s oceans, and this kept atmospheric CO2 levels low,” details McQuaid. “However, some recent research analyzing ocean sediment cores has shown that high levels of iron-rich dust don’t necessarily correlate with high growth rates of phytoplankton. This has led paleobiologists to speculate whether something else could be controlling the growth of phytoplankton.”
By 2008, the team was on the trail of several genetic clues. Senior author and biologist Andrew E. Allen identified several genes in diatoms that were responsive to iron, yet had no known function. At around the same time, McQuaid was subjecting plankton found near Antarctica to DNA analysis. McQuaid found every major Southern Ocean phytoplankton group had a copy of one of the genes Allen identified: ISIP2A.
However, this presented a new riddle for the scientists. Previous research suggested that phytotransferrin, a transferrin-like protein, was indispensable in the marine environment, but ISIP2A and transferrin didn’t resemble each other in the least. The team put the new discipline of synthetic biology to this test, using it to prove that ISIP2A was in fact a kind of transferrin.
Synthetic biology and the “Frankendiatom”
In the interdisciplinary field of synthetic biology, researchers combine engineering and biological techniques to solve research problems.
“We didn’t have a crystal structure of phytotransferrin, but we were able to show it was a transferrin through evolution, complementation (swapping out phytotransferrin with human transferrin inside a diatom), and by demonstrating the synergistic binding interaction—which is entirely unique to the mechanism of transferrin,” states McQuaid.
In the case of this phytoplankton study, the team first created a “knockout marine diatom” from which they deleted the ISIP2A gene. They then created a synthetic human transferrin gene, and inserted it into the “knockout” marine diatom, forming a “Frankendiatom” of sorts. The goal was to demonstrate that ISIP2A was a kind of transferrin.
“When you have an unknown protein that you suspect has a particular function, the ‘gold standard’ in biology is delete it and then replace it with a protein of known function,” explains McQuaid. “This is routine in mice (‘knockout mice’), but this has never been done in a marine organism, so we had to develop tools from scratch. We figured out how to delete diatom genes and then how to build artificial chromosomes and get bacteria to inject them back into diatoms—this is some really bizarre biology. We built a Frankendiatom: we deleted the gene for ISIP2A, and when we replaced it with a synthetic human transferrin gene, our Frankendiatom sputtered back to life, acquiring iron as before and providing really strong evidence that the mystery gene was indeed phytotransferrin.”
This was critical to understanding the issue here, because it means that both iron and carbonate have to be present for phytoplankton to thrive. Carbonate and iron bind simultaneously in transferrin; one cannot bind without the other. This team hypothesized that phytotransferrin in diatoms also engage in this unique style of synergistic binding, and that due to this synergy, fewer carbonate ions might slow the growth of phytoplankton.

Andrew Allen prepares a sample of phytoplankton filtered from the Ross Sea. In several of the Antarctic marine samples, phytotransferrin was among the most abundant proteins detected. (Credit: E. Bertrand)
“Phytotransferrin, and the transferrin mechanism in general, is fairly unique in the biochemical world: both substrates have to bind synergistically, which is a really unusual way for a protein to function,” adds McQuaid. “Normally for a biochemical reaction involving two substrates, you bind one, then the other. The need to bind both at the same time means there is an absolute proportionality between the concentration of iron, the concentration of carbonate, and the rate at which it can be bound. It is rare to find such a clear linear relationship.”
Proving the point was no easy task. Replicating an iron-poor underwater environment on land is more difficult than it may sound. Iron is the fourth most common element in the Earth’s crust, and the most common element on Earth by mass, making up a large portion of Earth’s inner and outer core.
“Modern industrial processes use steel to fabricate nearly everything, so iron contaminates all reagents, labware, and surfaces,” remarks McQuaid. “Here on land, iron drifts around as dust, clings to our clothes, and gets tracked around on our shoes. To drive our iron concentrations down to what you might see way out in the dust-free open ocean—in the trillionths of grams per liter—we had to do all of our experimentation inside a class-100 cleanroom and wear special dust-free clothing, similar to what you might see in a nanofabrication facility.”
Environmental implications, past and future
Upon exploring the evolutionary paths of phytotransferrin and transferrin, the researchers were surprised to learn that the proteins both originated about 700 million years ago in the pre-Cambrian realm of the Earth. The two functioned as analogs, and both appeared on the planet before either modern plants or animals—and before the five mass extinction events of Earth’s past. The appearance of phytotransferrin roughly corresponds with a period in Earth’s history characterized by massive changes in ocean chemistry, which explains in part why transferrin and ISIP2A have not been connected in the past.
The finding that the mechanism for acquiring iron in phytoplankton requires carbonate ions sheds new light on past mass extinctions—and on the prospect of climate change now. Rising atmospheric CO2 levels are triggering ocean acidification which is in turn decreasing carbonate levels. In fact, the concentration of surface carbonate ions in the world’s oceans is likely to decrease by 50 percent by 2100 under a “business as usual” (BAU) climate change model. Bioavailability of both carbonate and iron will control whether the phytoplankton at the base of the marine food web collapses or survives.
“It isn’t just the amount of iron in the ocean which may be controlling growth, but also the ability to access it,” asserts McQuaid. “It is not just the concentration, but the bioavailability of iron, and that bioavailability is directly proportional to the concentration of carbonate (which is in itself proportional to the concentration of atmospheric CO2).”
In other words, the research has shown how reduced bioavailability of carbonate caused by excess CO2 in the atmosphere that acidifies the ocean controls the growth of phytoplankton by preventing sufficient iron uptake. If the concentration of sea surface carbonate ions decreases by 50 percent by the end of this century as predicted, the effects we can expect to see are likely to be serious.
“Accessing alternate forms of iron rather than the free or ‘bioavailable’ iron is generally more difficult because uptake rates are slower, so a decrease in carbonate ions will likely affect organisms such as diatoms which rely on phytotransferrin negatively, and select for other organisms,” McQuaid states. “Thus there will be winners and losers in a high-CO2 planet, and we don’t necessarily know who that will be; this is one of the risks of large scale changes to the marine environment. The fossil record however, seems to show us that there will potentially be a lot of losers.”
While other research has shown that ocean acidification is harmful to corals, oysters, and other marine species, the finding that planktonic marine organisms must have carbonate ions for iron uptake, and that more plentiful bicarbonate can’t serve in its place, is new and concerning information.

Transferrin and phytotransferrin are functional analogues with a common origin. (Credit: McQuaid et al)
“This is all hypothetical, and it will take years of experimentation to determine how much of an effect the phytotransferrin-carbonate connection will have on ocean productivity,” cautions McQuaid. “Interestingly, the rapid injection of CO2 in the atmosphere has been tied to several of the earth’s mass extinction events, and these extinctions manifest themselves with particular intensity in the marine environment. In the paper we dated the appearance of phytotransferrin to 940 to 671 million years ago, thus this protein has been around could have been a contributing factor in extinctions—it has certainly been around long enough.”
The Paleocene-Eocene Thermal Maximum (PETM) is of particular interest in this context. In this mass extinction event, volcanism may have injected up to 1 billion pounds of CO2 into the Earth’s atmosphere, causing rapid ocean acidification, numerous extinctions, and a tremendous reordering of marine ecosystems. The team’s findings highlight one possible mechanism that might have driven this process. Notably, McQuaid points out, “we are currently adding 11 billion pounds [of CO2 into the Earth’s atmosphere] per year, or about 10 times the rate of that particular extinction cycle!”
The significance of ISIP2A also being in diatoms is linked to their carbon sinking behavior.
“Diatoms have heavy shells made out of silica, thus they are good ‘sinkers’, meaning that after they die, they slowly sink and drag their fixed carbon down with them into the depths of the ocean, and therefore remove (or sequester) carbon dioxide from the atmosphere,” explains McQuaid. “They are a literal sink for CO2! Land plants absorb CO2 when they grow, but most of that CO2 is returned to the atmosphere when the plants die and decompose. With diatoms, if that carbon reaches the bottom of the ocean, it is generally sequestered from the atmosphere for long periods of time (centuries to millennia).”
Among the other fascinating points from this research, the idea that iron uptake is a tricky process on our planet is hard to miss.
“We find it fascinating that nature rediscovered the same design: phytotransferrin and transferrin are analogs which arose through convergent evolution,” McQuaid adds. “The fact that the same carbonate binding mechanism keeps reappearing seems to suggest there are limited methods to lock onto iron in the marine environment.”




0 comments